Otaigbe iowa state university goals of this unit to compare and contrast the structures of metal ceramics and polymer materials explain the three most important structures for metals describe factors affecting crystal structure in ceramics define polymorphism.
Structures of metals and ceramics.
Structures of metals and ceramics.
More complex than metals since two or more elements present bonding can vary from purely ionic to purely covalent and many in between if dominantly ionic bonding crystal structures have electrically charged ions instead of atoms cations positively charged lost electrons anions negatively charged gained electrons.
Recall that when metal in the liquid state is cooled a crystalline solid precipitates when the melting freezing point is reached.
Metal and ceramic crystal structures instructor.
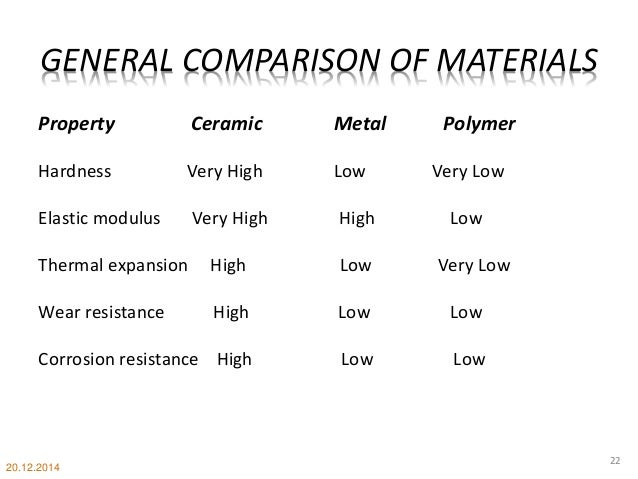
The table below provides a summary of the main properties of ceramics and glass.
Start studying ch 3.
For metals the chemical bond is called the metallic bond.
Today we ll explore more about two of the three main types of materials that we use as engineers.
Ceramic structures continued ceramic glass ceramics with an entirely glassy structure have certain properties that are quite different from those of metals.
Amorphous structure means that atoms are not organized according to a well ordered repeating arrangement as in crystals.
Metals many ceramics some polymers atoms have no periodic packing occurs for.
Industrial ceramics are commonly understood to be all industrially used materials that are inorganic nonmetallic solids.
Materials science engineering ceramic crystal structure 3 3 timeline ce.
The atoms in ceramic materials are held together by a chemical bond.
Learn vocabulary terms and more with flashcards games and other study tools.
Ceramic composition and properties atomic and molecular nature of ceramic materials and their resulting characteristics and performance in industrial applications.
Usually they are metal oxides that is compounds of metallic elements and oxygen but many ceramics.
The bonding of atoms together is much stronger in covalent and ionic bonding than in metallic.
Noncrystalline materials complex structures rapid cooling si oxygen crystalline sio2 amorphous noncrystalline noncrystalline sio2 adapted from fig.